Do We Need to Revisit Causality?
The recent release of Pfizer's clinical trial data may shed new light.
For over a year now I’ve been watching regulatory authorities and “fact-checkers” dismiss the staggering number of heart attacks, strokes, miscarriages, neurological issues and deaths that have been occurred after COVID-19 injections.
“Correlation is not causation” is the mantra that is used when “safe and effective” is challenged by the timeliness of these serious adverse events and deaths.
And there are more than just a few. According to VAERS, there have been over 783,000 adverse events and 11,500 reported deaths following COVID-19 shots as of March 4.
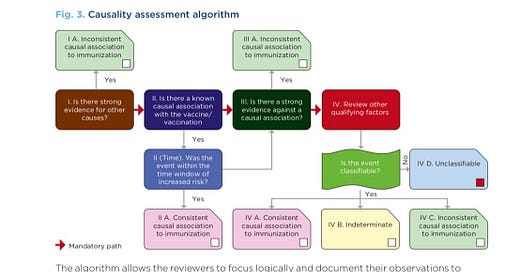
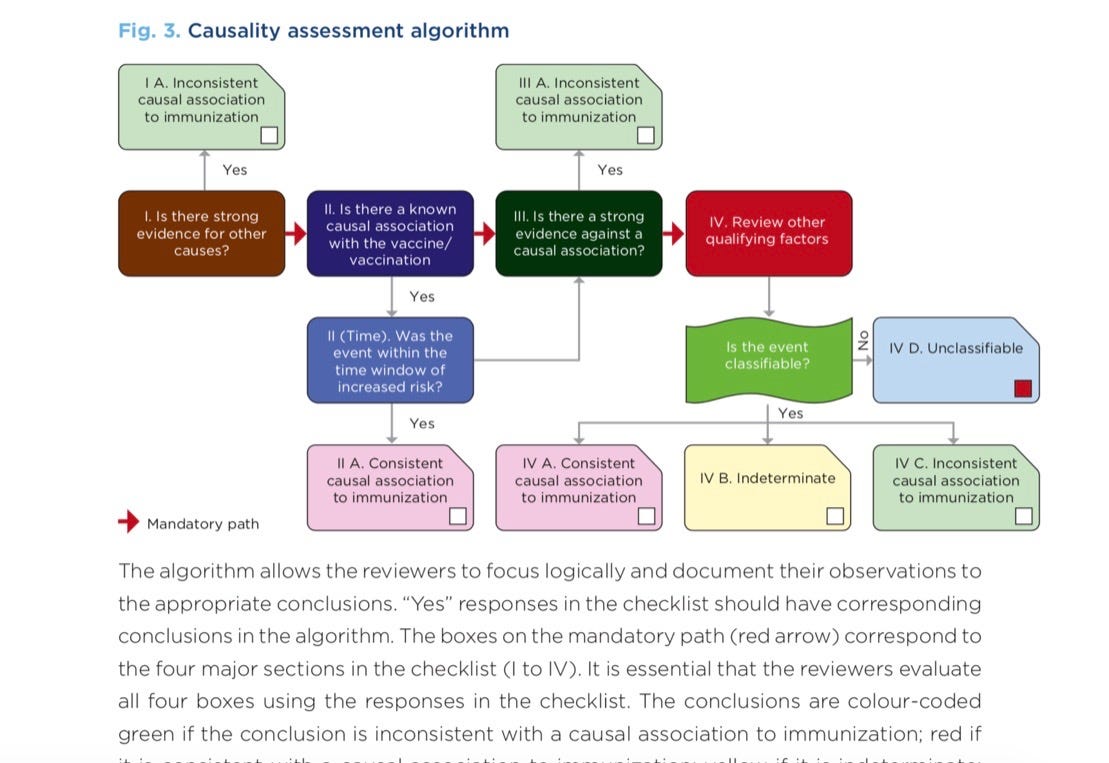
Unfortunately, the WHO Causality Assessment of an Adverse Event Following Immunization (AEFI) algorithm makes it virtually impossible to determine causality.
“In the new causality assessment, only reactions that have previously been acknowledged in epidemiological studies to be caused by the vaccine, are classified as a vaccine-product–related-reactions. Reactions observed for the first time during post-marketing surveillance (Phase 4 clinical trial) are not considered as ‘consistent with causal association with vaccine’. All new serious adverse reactions are labelled as coincidental events ‘inconsistent with causal association,’ or ‘unclassifiable’ and the association with vaccine is not acknowledged. (5). It has, in effect, made phase 4 trials redundant.”
https://www.bmj.com/content/365/bmj.l2268/rr-0
Last week, new information from Pfizer’s clinical trial data was released to the public, which includes 9 pages of “Adverse Events of Special Interest.”


While the list apparently represents adverse events that must be monitored and not necessarily events that have occurred, we do not yet know which of these have turned up in clinical trial data.
It’s now a waiting game… but this is far from over.
Stay tuned.